Technical Capabilities
We’re dedicated to excellence in research & development, manufacturing, in-depth testing, efficient packaging, and personalized service. We are committed to improving existing products and adding new products. We can assist your company in expanding your product line as well.

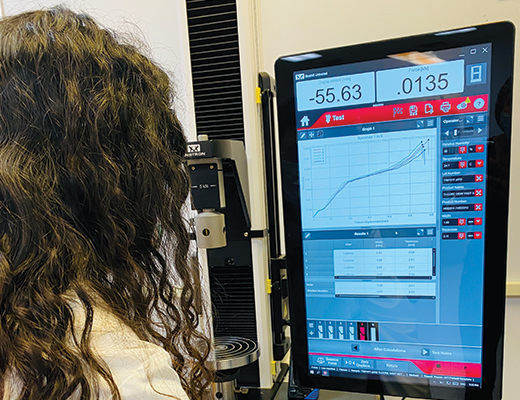
Research & Development

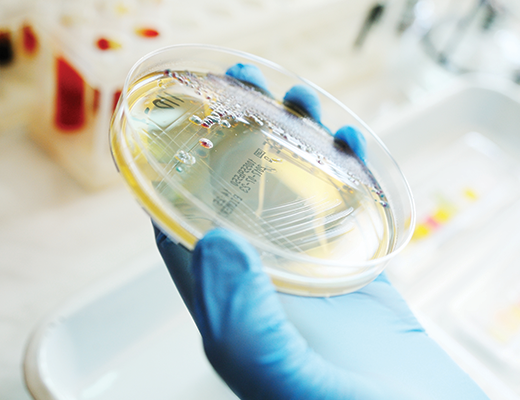
Microbiology

Manufacturing Excellence
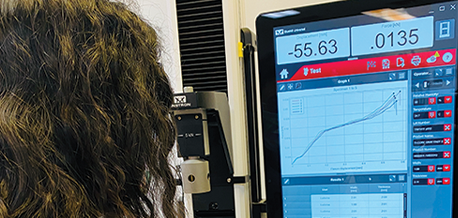
Analytical Testing

Quality Control

Regulatory Affairs

Serialization

Stability Testing